Anermo Capsule
Manufacturer: Gansu Hexi Pharmaceutical Co., Ltd.
Approval number: Zhunzi Z20090255
Illegal facts: In advertisements, it is declared that “there are significant effects on neurasthenia, menopausal syndrome, and the symptoms of low immunity in the elderly, and no adverse reactions are seen in tens of thousands of clinical cases.â€
The drug is a prescription drug and advertising on the mass media is prohibited. The advertising of product advertisements exceeds the contents of approval of the indications of product functions, contains assertions and guarantees for expressing efficacy, and uses the names and images of state agencies, professional agencies and their staff members and patients to prove the efficacy of products.
Shujin Huoli Wan
Manufacturer: Liaoning Huayuan Tianli Pharmaceutical Co., Ltd.
Approval number: Zhunzi Z21020265
Illegal facts: The advertisement declares "not a pain killer, but it is even better than a pain killer ... to get the patient out of the pain of repeated treatment and to avoid surgery and squeaking." The advertisement of the product contains unscientific assertions or guarantees that express efficacy, and uses the names of medical institutions, scientific researchers, and patients as proofs to seriously deceive and mislead consumers.
Oolong Yangxue Capsule
Manufacturer: Hulunbeier Songlu Pharmaceutical Co., Ltd.
Approval number: Zhunzi B20020971
Illegal facts: The ad declares that “the patients with coronary heart disease take 5 boxes of heart to feel no panic, no shortness of breath... ...take 10 boxes of basic improvement...take 20 boxes of heartbeat and normal pulse...â€
The advertisement of the product contains unscientific assertions or guarantees that express efficacy, and uses the names of medical institutions, scientific researchers, and patients as proofs to seriously deceive and mislead consumers.
Forefront of tea
Manufacturer: Guangxi Pingnan Pharmaceutical Factory
Approval number: Zhunzi B20050023
Illegal facts: The advertisement declares that "the national secret formula of Chinese medicine, important science and technology awards, the country's exclusive production ... State Food and Drug Administration approved ..." and so on. The advertisement of the product contains unscientific assertions or guarantees that express efficacy, and uses the names of medical institutions, scientific researchers, and patients as proofs to seriously deceive and mislead consumers.
Hypoglycemic clearing paste
Manufacturer: Jiyuan Taiji Medical Products Co., Ltd.
Medical Device Registration No.: Yu Ji Food and Drug Supervision (Quasi) Word 2009 No. 1640001
Illegal facts: In advertisements, it is declared that “do not take medicine without injections, curb complications... State-owned drug administration departments are highly recognized...core technology is at the forefront of the world...†and so on. The advertisement of this product contains content that uses medical research institutes, academic institutions, medical institutions or specialist doctors, patients' names, images, etc. as proof.
Feng Yuan Lu Lu San Qi Alixa Tea
Manufacturer: Beijing Lierkang Medical Technology Development Center
Approval number: Guoshi Jianzi G20060089
Illegal facts: The advertisement declares that the product “eradicate high blood pressure, stop the drug for 30 days, reduce blood fat and make up the kidneys, ... the heart becomes young, and eradicate gastrointestinal diseases†and so on.
The health product advertisement contains an unscientific assertion or guarantee of efficacy, exaggerates the efficacy of the health food, expresses or implies that the health food has the role of disease treatment by publicizing the functions of certain ingredients, and utilizes state organs, professional organizations, and their staff. Proof on behalf of the name, seriously deceive and mislead consumers.
Jiaxin Haopai Chili Film
Manufacturer: Beijing Jiaxin Biomedical Technology Co., Ltd.
Approval number: Guoshi Jianzi G20050393
Illegal facts: The advertisement declares that the product “eliminates nephrotoxicity, live kidney blood...takes 30 days of weakness, weak waist and knees are all eliminated...†and so on.
The health product advertisement contains an unscientific assertion or guarantee that expresses efficacy, exaggerates the efficacy of the health food, expresses or implies that the health food has the role of disease treatment by propagating certain components, and uses the name of the expert and the consumer as proof. Seriously deceives and misleads consumers.
Brain plug pills
Manufacturer: Jilin Special Research Pharmaceutical Co., Ltd.
Approval number: Zhunzi Z62021304
Illegal facts: The advertisement declares "a box of effective, invalid refunds ... 71 patented technologies, professional treatment of cerebral embolism ...". The drug is a prescription drug and advertising on the mass media is prohibited. The advertising of the product's functional indications exceeds the contents of the approval, contains assertions and guarantees that express efficacy, and uses the names and images of state agencies, professional organizations, their staff, and patients to prove the efficacy of the products.
Electric Curtains And Curtain Motor
Person can control the curtains open or close with switch.
Electric curtain machine consists of three parts:
1. Intelligent control system for controlling the opening and closing of curtains.
2. Operate and set the main controller of the wireless controller. (electric curtain)
3.Pulling the curtain actuator motor and pulling mechanism. The host machine controls the positive and reverse rotation of the motor, and the curtain is automatically closed or opened by a pulley.
It can make the infrared remote control the curtain open or close the directly,setting up the main controller automatic function with remote control, once set up and open the automatic control function, the main controller will automatically control function according to it.
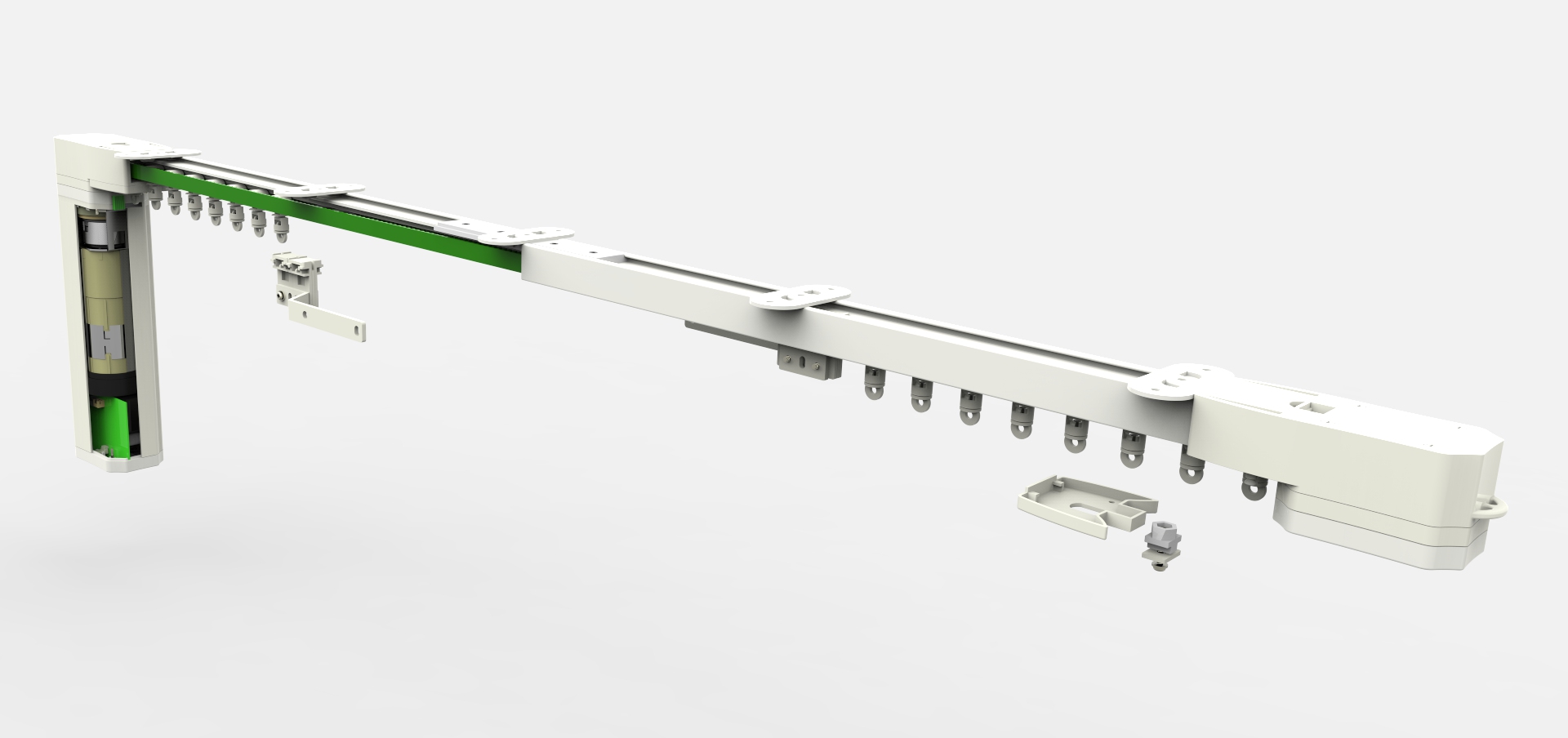
Electric Curtains,Curtain Motor,Smart Curtains,Electric Curtain Motor
Shenzhen Zhuohao Intelligent Electronic Development Co., Ltd. , https://www.szactop-smart.com
