Reduce the migraine time by half! Amgen's new drug is expected to be approved next year
December 01, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Amgen announced today the positive results of a six-month, phase 3 clinical trial of STRIVE in the New England Journal of Medicine, which evaluated Aimovig (erenumab) versus placebo for paroxysmal migraine Prevention of 4 to 14 migraine days per month. Aimovig showed statistically significant statistically significant results at all major and secondary endpoints in the study.

People with frequent migraine headaches may have headaches for half of their lives. Migraines take away the time patients spend with their families, productivity at home and at work, and even life. Migraine suffers from debilitating pain, physical damage, and fear of the next episode. The World Health Organization ranks migraine as one of the most debilitating diseases. Ten million Americans with frequent migraine suffer severely from their daily lives. Preventive drugs are an option, and about 3.5 million of these patients are currently receiving preventive treatment, but 80% stop taking them within a year. Migraine brings pain, disability and financial burden to individuals and society, and is still not recognized and treated.
Aimovig is a treatment for preventing migraine by blocking CGRP receptors associated with migraine activation. Aimovig has been studied in several large, global, randomized, double-blind, placebo-controlled studies to assess its safety and efficacy in migraine prophylaxis. More than 2,600 patients participated in the Aimovig clinical program in four placebo-controlled Phase 2 and Phase 3 clinical studies and their open-label extension studies. Regulatory applications have been filed in the US and Europe. The FDA's target approval date (PDUFA date) is May 17, 2018.
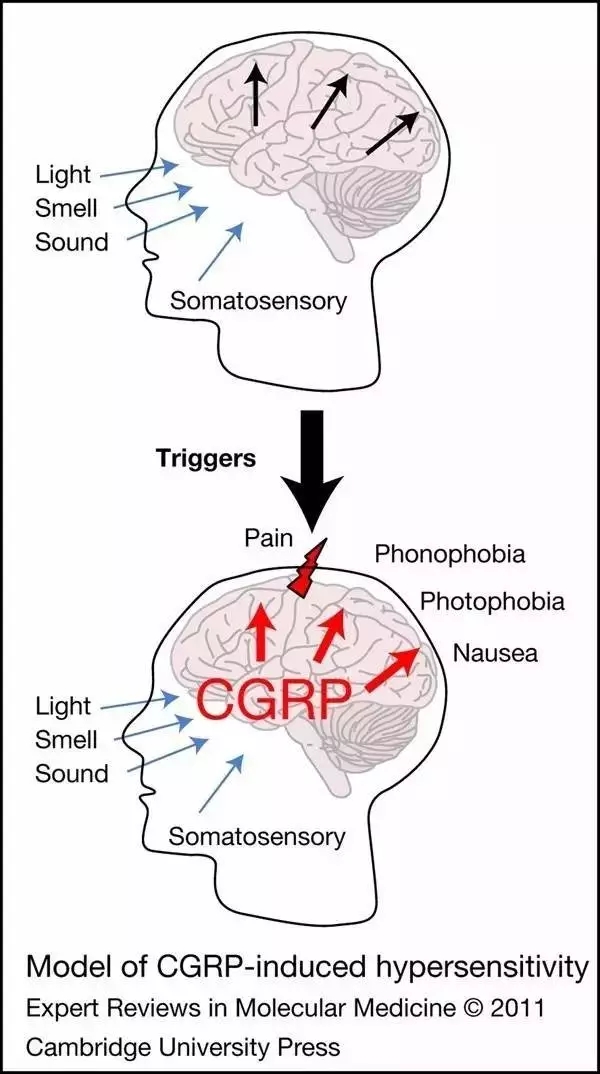
â–²The principle of CGRP leading to migraine (Source: Molecular Medicine)
STRIVE (Research Assessment of Erenumab Efficacy and Safety in Migraine Prevention, NCT02456740) is a global 3-phase, multicenter, randomized, 24-week, double-blind, placebo-controlled study evaluating Aimovig for the prevention of paroxysmal migraine Safety and effectiveness of an average of 4 to 15 migraine days per month for the first three months of screening. In this study, 955 patients received a monthly subcutaneous injection of placebo, or Aimovig (70 mg or 140 mg), at a random ratio of 1:1:1. Patients experience 4 to 15 migraine days per month with a baseline of 8.3 migraine days per month. The primary endpoint was the last three months of the double-blind treatment phase of the study (Days 4, 5, and June), with an average monthly migraine days varying from baseline. Secondary study endpoints included an average monthly migraine reduction of at least 50% from baseline, an average monthly acute migraine-specific drug use days, and an average daily activity impact and migraine body function impact log (MPFID) at 6 months. The average body damage score.
Studies have shown that patients taking higher doses of Aimovig (140 mg) have a reduced migraine day of 3.7 days per month (3.2 days in the 70 mg group and 1.8 days in the placebo group, p < 0.001 for both doses compared with placebo) . 50% of patients taking 140 mg of Aimovig had a 50% or more migraine reduction, and the likelihood of achieving this goal was significantly higher than placebo (43.3% in the 70 mg group and 26.6% in the placebo group). The ratios compared to placebo were 2.8 and 2.1, respectively, p < 0.001).
Other secondary endpoint outcomes in Phase 3 clinical trials included a significant reduction in the number of days of acute or first-aid migraine-specific medications in patients taking Aimovig compared with patients taking placebo (140 mg reduction in 1.6 mg per month and reduction in 1.1 mg in the 70 mg group) On days, the placebo group was reduced by 0.2 days, p < 0.001). Aimovig reduced the impact on daily activities in migraine patients (5.9 points in the 140 mg group, 5.5 points in the 70 mg group, and 3.3 points in the placebo group, p < 0.001). In addition, physical impairment scores were also significantly reduced (4.4 points in the 140 group, 4.2 points in the 70 mg group, and 2.4 points in the placebo group, p < 0.001). In the STRIVE study, Aimovig's overall safety and tolerability were similar to placebo.
“STRIVE is the first phase 3 clinical study to fully report monoclonal antibodies to the CGRP pathway, and it clearly shows that blocking this pathway can reduce the impact of migraine,†said Dr. Peter Goadsby, professor of neurology at King's College London, London. The results represent a true shift in the treatment of migraine patients, from a treatment that does not understand migraine to a specific design for migraine treatment, STRIVE and monoclonal antibody development, representing the understanding of migraine principles and bias A very important step in the treatment of headaches."

â–² Dr. Sean E. Harper, Executive Vice President of R&D, Anjin (Source: Anjin official website)
“There are clear unmet medical needs today that require effective innovative therapies to prevent migraine. The publication of these data highlights the CGRP receptor blocker Aimovig as the potential first available treatment that targets pathophysiological related pathways to address One of the most common causes of disability in the world," said Dr. Sean E. Harper, executive vice president of research and development at Amgen. "We look forward to advancing a strong clinical program for Aimovig to help alleviate this devastating disease. Burden and best support the migraine patient population."
Phase 3 clinical trials The STRIVE study is one of the key studies that Aimovig is undergoing regulatory review in the US and Europe. If approved, Amgen and Novartis will jointly commercialize Aimovig in the United States. We look forward to the successful review of this new drug to relieve headaches and improve the quality of life of patients.
Reference materials:
[1] Mind-Blowing: Amgen's Aimovig Halves Length of Migraine Attacks in Phase III Study
[2] AimovigTM (erenumab) Phase 3 STRIVE Data Published In The New England Journal Of Medicine Demonstrate Significant, Sustained Efficacy In Migraine Prevention
Laxative Powder,Laxaday Powder,Smooth Lax Powder,Constipation Relief Powder
Shaanxi Zhongyi Kangjian Biotechnology Co.,Ltd , https://www.zhongyiherbs.com
